The update to the ICH GCP E6 (R3) guideline is currently in its final stages. The guideline is being updated in 2 parts: First the Principles and Annex I are being updated and will go live, secondly Annex II will be released. The timelines of the ICH GCP R3 update was discussed in the ICH Assembly meeting in Montreal. If the discussed timelines are met, the updated Good Clinical Practice Principles and Annex I will be adopted early 2025 and Annex II later in 2025.
In this article, we have incorporated the status of the documents that form the latest version of ICH GCP, known as R3, in the ICH formal procedure steps to update guidelines. In previous blogs, we’ve covered the upcoming changes that are coming to the Principles. For more details on the revised content of the Principles, Annex 1 and Annex 2, please read part 1 and part 2 of our ICH GCP R3: What Should We Expect? Series.
Read about the process for adopting guidelines in this blog, to learn more about the timelines and be prepared for the ICH GCP E6 (R3) update.
ICH Formal Procedure Steps for Updates on Guidelines
The ICH Formal Procedure for adopting a new guideline involves five structured steps designed to ensure thorough development, consensus, and harmonization.
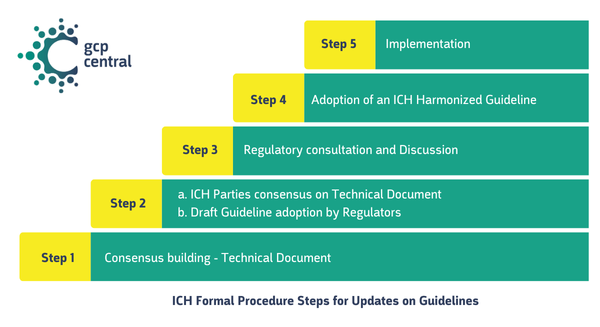
Spoiler alert: ICH-GCP R3 Principles and Annex I is currently in Step 4, where ICH-GCP R3 Annex II is in Step 3. Let’s take a look at the steps.
Step 1: Consensus Building
The Expert Working Group (EWG) develops a draft Technical Document, based on the Concept Paper and Business Plan approved by the ICH Assembly.
The EWG communicates via various means, including face-to-face meetings at biannual Assembly meetings, to reach consensus.
Once consensus is reached, the draft is submitted to the Assembly for adoption under Step 2.
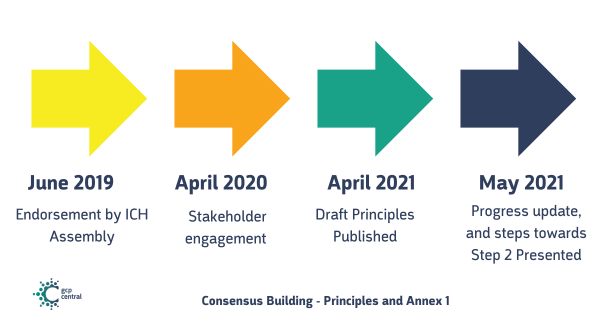
Principles and Annex 1
Step 1 started with an endorsement by the ICH Assembly in June 2019. A session was organized in April 2020 to engage stakeholders resulting in Draft Principles being published in April 2021. During a global web conference in May 2021 an update on the progress of the draft Principles was presented, as steps towards Step 2.
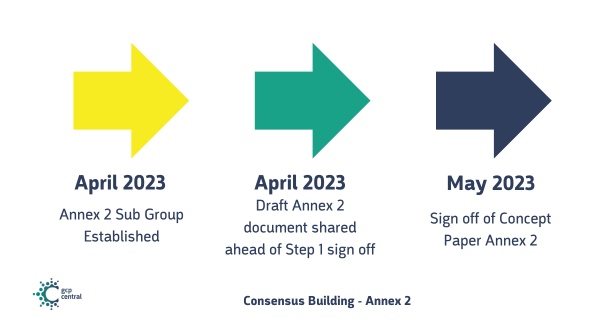
Annex 2
The annex 2 sub-group was established in April 2023, The draft Annex 2 document was shared in April 2024 ahead of Step 1 sign-off in May 2023. The Annex 2 Concept Paper was approved in May 2023 by the ICH, and at the same time an E6(R3) EWG Annex 2 Sub-group was established to begin development of Annex 2.
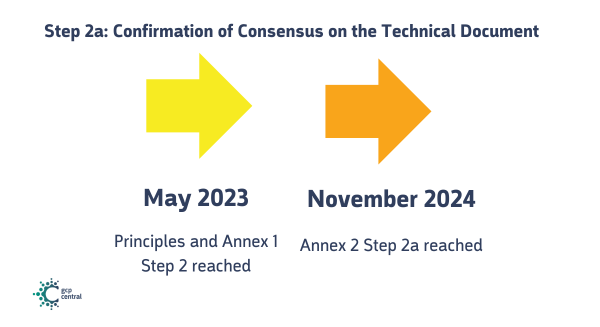
Step 2a: Confirmation of Consensus on the Technical Document
The ICH Assembly reviews the draft and ensures there is sufficient scientific consensus for the document to proceed to regulatory consultation.
Step 2a: Confirmation of Consensus on the Technical Document
The ICH Assembly reviews the draft and ensures there is sufficient scientific consensus for the document to proceed to regulatory consultation.
Principles and Annex 1
- Step 2 was reached in May 2023.
Annex 2
- Step 2a of Annex 2 was reached in November 2024.
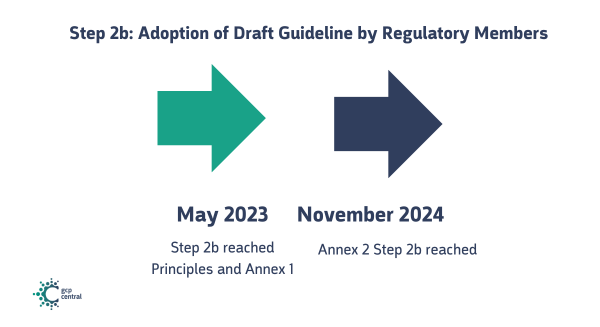
Step 2b: Adoption of Draft Guideline by Regulatory Members
Regulatory Members of the ICH review and endorse the draft guideline, allowing it to proceed to the consultation phase.
Principles and Annex 1
- Step 2b was reached on 19 May 2023
Annex 2
- Step 2b of Annex 2 was reached on November 22, 2024.
Step 3: Regulatory Consultation and Discussion
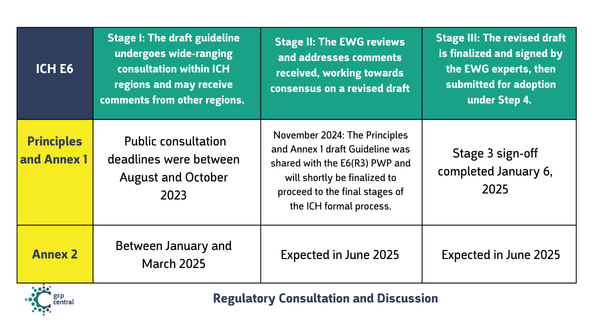
Stage I: The draft guideline undergoes wide-ranging consultation within ICH regions and may receive comments from other regions.
Stage II: The EWG reviews and addresses comments received, working towards consensus on a revised draft.
Stage III: The revised draft is finalized and signed by the EWG experts, then submitted for adoption under Step 4.
Principles and Annex 1
Stage I: Public consultation deadlines were between August and October 2023.
Stage II: November 2024: The Principles and Annex 1 draft Guideline was shared with the E6(R3) PWP and will shortly be finalized to proceed to the final stages of the ICH formal process
Stage III: : Step 3 sign-off completed January 6, 2025.
Annex 2
Stage I deadlines = between January and March 2025
Stage II: Expected in June 2025
Stage III: Expected in June 2025
Step 4: Adoption of an ICH Harmonized Guideline
The ICH Assembly reviews and adopts the finalized guideline, confirming sufficient consensus among all members.

Principles and Annex 1
Step 4 was delivered in January 2025.
Annex 2
Expected in June 2025
Step 5: Implementation
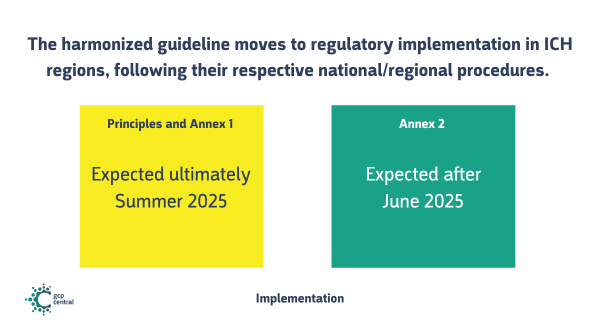
The harmonized guideline moves to regulatory implementation in ICH regions, following their respective national/regional procedures. Timelines vary from a timeframe of 1 month or a couple of months, depending on the region.
Principles and Annex 1
ICH does not mention an expected date for Step 5.
Annex 2
When step 3 and 4 are indeed reached according to plan in June 2025, this allows for regional implementation to commence in the Summer of 2025,
The European Medicines Agency (EMA) has announced the adoption of the ICH E6(R3) Guideline for Good Clinical Practice (GCP) The Guideline will be effective from June 2025, and adopted on the 23rd of July. ICH E6 (R2) remains in effect until 11 June 2025. This gives stakeholders time to transition to the new version, while still adhering to the previous standards.
Implications for Implementation
Once adopted, the ICH GCP E6 (R3) guideline will be ready for implementation in the various ICH regions. Historical data shows that the timeframe between adoption and implementation can vary widely—from as short as one month, as seen in the EU with the last ICH-GCP R2 update, to several years.
This structured and collaborative approach ensures that the new ICH GCP E6 (R3) guidelines will be harmonized across regions, facilitating a unified standard for good clinical practice globally.
GCP Central is dedicated to bring you the required knowledge to be ready for this ICH-GCP revision ahead of go live of the guideline. We launch our ICH-GCP R3 Transition training in February 2025, so sign up to pre-register here.
As updates are made to the guideline, GCP Central is committed to bringing these to you as soon as they are available via our range of ICH-GCP training (Including WMO GCP for those working in research in the Netherlands). Keep your training up to date, on the go with our myGCP platform.
Source:
ICH Assembly Meeting Agenda, 4-5 June 2024 Fukuoka, Japan.


