This is the first in a series of articles where GCP Central goes in-depth into the challenges, learnings, and critical findings of implementing the new European Clinical Trials Regulation (EU CTR). In this instalment, we reflect on the Clinical Trials Information System (CTIS), the unique single-entry point and database for clinical trials, and the issues surrounding the slower system adoption.
EU CTR Ushers In A Period Of Change
The introduction of the EU CTR in January 2022 saw a new era of simplifying and harmonizing clinical trials within the European Union (EU). This new regulation replaced the current directive, the EU-CTD. For the time being, the transition period from EU CTD to EU CTR means that entries of new clinical trial applications into CTIS are optional, but as of January 31st, 2023, the use of CTIS for your submission becomes mandatory.
The implementation of EU CTR and the use of CTIS has initially been slow. However, it has been picking up since the European summer. This is shown in the EMA report “Key performance indicators (KPIs) to monitor the European clinical trials environment” – version 5 was published in September. This report is produced as part of the Accelerating Clinical Trials EU (ACT EU) ‘s business program, and one of the priorities of this initiative is monitoring the implementation of the EU CTR.
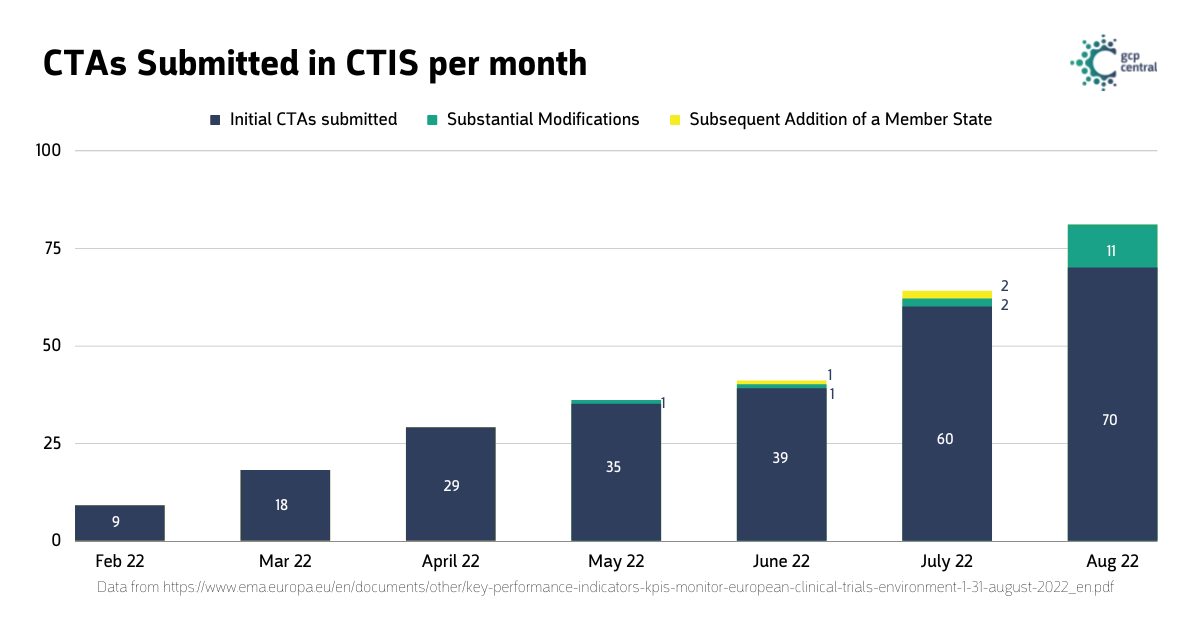
Overall, 278 clinical trial applications have been submitted to CTIS since the system’s launch on January 31st, 2022. 260 are initial clinical trial applications, 15 are substantial modification applications, and 3 are applications for adding a new Member State concerned.
CTIS Statistics Since Implementation
During August of the submitted applications, 10 were re-submissions, 7 were previously lapsed applications, and 3 were previously withdrawn applications. On average, it took the authorities of the Member States 77 calendar days to issue a decision; the fastest was 5 days, and the slowest was 118 days.
As of August 31st, 2022, 12 clinical trials have been authorized in at least one Member State Concerned and have started the recruitment of patients at their respective sites. European clinical trials submitted via CTIS that have a decision can be found on the publicly available website of EMA: https://euclinicaltrials.eu/home
Top 3 reporting Member States:
As a sponsor of a multinational trial, you can indicate your preference for a reporting Member State: the Member State that coordinates the review of your trial. In the case of a national trial, the country where the trial is conducted automatically is the reporting Member State. The EMA report shows that the following Member States are the 3 Top reporting Member States:
- Spain
- Denmark
- Germany
When looking at the amount of initial clinical trial applications with full dossier per Member State, the Top 5 are somewhat different:
- France (90 applications)
- Spain (82 applications)
- Germany (70 applications)
- Denmark (65 applications)
- Italy (57 applications)
It seems that however many trials are conducted in France and Italy, these countries are not chosen often as a reporting Member State.
Challenges of CTIS
The slow adoption of CTIS has seen some challenges concerning trial submissions. Some companies view 2022 as the last year they can submit under the CTD rather than taking the leap into a new regulation. However, the transition period is drawing to a close; as of January 31st, 2023, all new submissions for clinical trials involving a medicinal product must be made via CTIS.
Does that date impact current trials that have been approved under the CTD?
Yes. For example, extending your trial to another member state for a clinical trial authorized under the CTD via a substantial amendment is only possible in the first year of the transition period. Adding a new member state after January 31st, 2023, can only be done when the clinical trial is transitioned to the CTR. After transitioning the trial, the addition of sites in a new country can be submitted via CTIS according to the procedure ‘Add new Member State.’
While the hesitancy about adopting a new system is valid, the earlier you adopt the use of CTIS, there is less stress involved with making changes to your clinical trial and creating unnecessary and costly delays.
What Solutions Are Available?
While introducing a new submission portal can be challenging, the good news is that there are options available for staff training and updating your understanding of both CTIS and the EU CTR.
Firstly, training on how to use the portal is available via the EMA, with free e-learning modules readily available. These modules are updated regularly, and the training covers the complete life cycle of clinical trial submission, authorization, and supervision.
Through our myGCP learning portal, you can also access staff training at expert level, or choose a role-based variation, with either classroom or blended training available on request. Our expert trainer, Marieke Meulemans, was involved in validating both the EMA’s CTIS training materials and was also a member of the Dutch EU CTR Steering Committee (DCRF), supporting the implementation of EU CTR in the Netherlands.
The myGCP learning portal has the convenience of on-the-go learning so that it can be completed in your own time, at your own pace. Important regulatory updates are directly implemented in your training, meaning you can be up to date with the latest changes to EU CTR as they happen.
We’ve also provided a list of important links and upcoming events at this article’s end, with essential training and education opportunities.
Are You EU CTR Ready?
Implementing the EU CTR and submitting your trial applications via CTIS doesn’t need to be challenging. GCP Central has your training needs covered with our EU CTR Expert course. With comprehensive insights into the requirements of the new European legislation for clinical drug research, you’ll be trials-ready before the deadline of January 31st, 2023.
For in-company training solutions, contact us via this link. GCP Central is committed to making sure you’re #EUCTRReady.
Important Links
Clinical Trials Regulation: Progress on Implementation – EMA
Clinical Trials Information System (CTIS) Webinar – 9 months on and going forward – EMA (November 16th, 2022 13:30 – 17:30 (fee applies) )
Clinical Trials Information System (CTIS) sponsor end-user training program – EMA (November 7-10, 2022 14:30 – 18:30 (fee applies) )



