Acknowledge your knowledge
We’re close to launching our new myGCP Powerapp and are incredibly excited about it. We’d love to give you some insight into the app’s development in this blog. Why create this app? Because we ran into an old, ingrained pattern and would like to break habits. Recognizing your knowledge of clinical research rules needs to be more efficient, which is why (the mandatory) GCP certification is woven into the system. How does the app work? We’re sharing the rationale behind building the app and giving a preview of the app environment. What are the user experiences with the app? We also engaged in continuous learning and, of course, conducted a few pilots. We would love to share the most striking and exciting results with you.
This 3-part series will take you through the app’s whys and hows and the pilot experiences. Let’s begin with part 1, it’s about our motivation for developing the app.
Part 1 – Why this app?
With the myGCP Powerapp, learning becomes even more efficient, unlike regular studying. Knowledge is retained, refreshed and tested over a more extended period. If you have already mastered specific learning through experience, such as the principles of Good Clinical Practice, this is recognized, and you move through the training faster. Moreover, you can go back and refresh a topic in a targeted way at a later date if you need it sooner. And in case of an inspection/audit, you can easily demonstrate that you are qualified and certified.
As a side effect of launching the myGCP Powerapp, we hope that a discussion will arise in the life science sector. This discussion should be about how we deal with acquiring and retaining knowledge, why we consider training more important than qualification and why we waste unnecessary time on training.
How do you feel about the following pattern? Training > knowledge > certificate > complete.
With the myGCP Powerapp, knowledge is offered and tested directly over a more extended period. This is done through question-based learning, lots of variation and repetition. Of course, the content of the Powerapp is based on the EMWO test requirements, and at the moment, you can choose from the topics WMO, ICH-GCP, EU CTR and GDPR. We are still experimenting with the requirements for certification. One idea is to issue a certificate at a score of a percentage of 80% or higher, held over a 4-week period. This demonstrable retention of knowledge over an extended period of time fits with our philosophy on learning, about which more later. However, the 4-week time period is open to debate. The same applies to the WMO/GCP re-registration requirements using the myGCP Powerapp. We are currently discussing this with EMWO, and hopefully, we can offer WMO/GCP re-registration via the app soon! This is because the app is also highly suitable for re-registration.
What we aim for with the introduction of the myGCP Powerapp, is to abandon an old mindset. The mindset where we only measure and certify on the element “training”. We measure existing knowledge and provide new knowledge, recognized as certificates. In the end, the certificates will have more value because of this.
After obtaining their certificate, the user can continue to learn through the app. This way, you can keep your knowledge of the different steps in clinical research up-to-date and refresh-specific knowledge if you need it soon. This allows continuous learning to continue after obtaining a certificate, an essential fact that fits our vision!
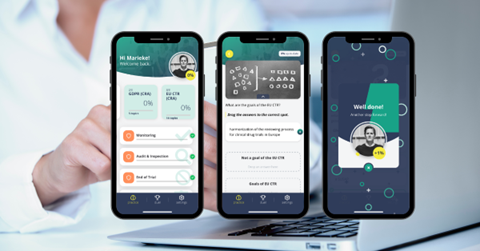
While using the Powerapp, you have continuous insight into your progress. In the pilots conducted, the app scored high on ‘the insight of knowledge level per subject’! This insight is excellent for you and also for us to be able to determine certification. With this, we then recognize your knowledge. That is what we have in mind with the myGCP Powerapp:
To acknowledge your knowledge!
We are very curious about your reaction to the above piece. Do you agree with abandoning old mindsets? Will you join us in discussing the length of time for certification and re-registration, or do you have a different idea? Tell us what you think on our LinkedIn page here.


