
by GCP Central | Dec 3, 2014 | GCP Central News
The Faculty of social and behavioural sciences, health-medical section and Neuropsychology (GMN) of Leiden University is working hard to set a quality standard for their research. GCP Central was invited to give a WMO / GCP training. Behavioral sciences research has...

by GCP Central | Nov 7, 2014 | GCP Central News
In mid-2016, the new European regulations will come into effect once the European database (the ‘portal’) is ready. From that moment on, the submission process for drug research will be aligned for all EU Member States. The idea is that the new regulation...

by GCP Central | Nov 6, 2014 | GCP Central News
In mid-2016, the new European regulations will come into effect once the European database (the ‘portal’) is ready. From that moment on, the submission process for drug research will be aligned for all EU Member States. The idea is that the new regulation...
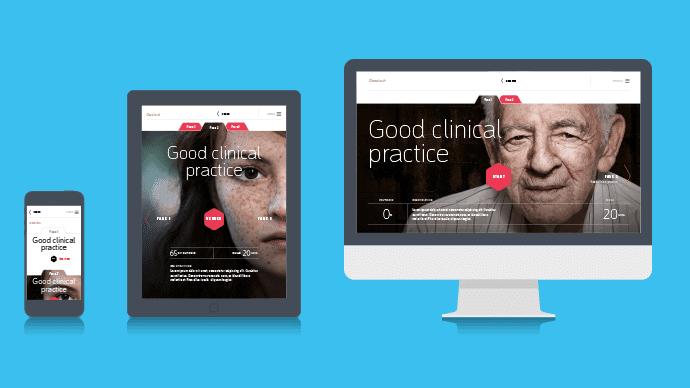
by GCP Central | Nov 3, 2014 | GCP Central News
Our learning environment is getting more and more shape! Together with our partner BeSpeak we will design a GCP platform where you will find GCP training, cases, news and GCP experiences of others. From February 2015 you can put together your own GCP training through...

by GCP Central | Nov 3, 2014 | Action
For the content of our GCP training we work together with doctors, researchers, medical directors, CRAs, auditors, research nurses and many others. Their experience, knowledge and daily dilemmas during clinical research, provide input to our cases and ensure that the...

by GCP Central | Sep 26, 2014 | GCP Central News
Founder of GCP Central, Marieke Meulemans, talks about the origin of the company. “In 2003 I started my working life at Kendle on the Department quality control (QC)I checked the quality of the data from the drug trials, the files were completely checked, etc....








