We are getting more and more questions about the EU CTR. The transition period is about to end, and we ‘re noticing it. That’s why we would like to share with you the most critical issues:
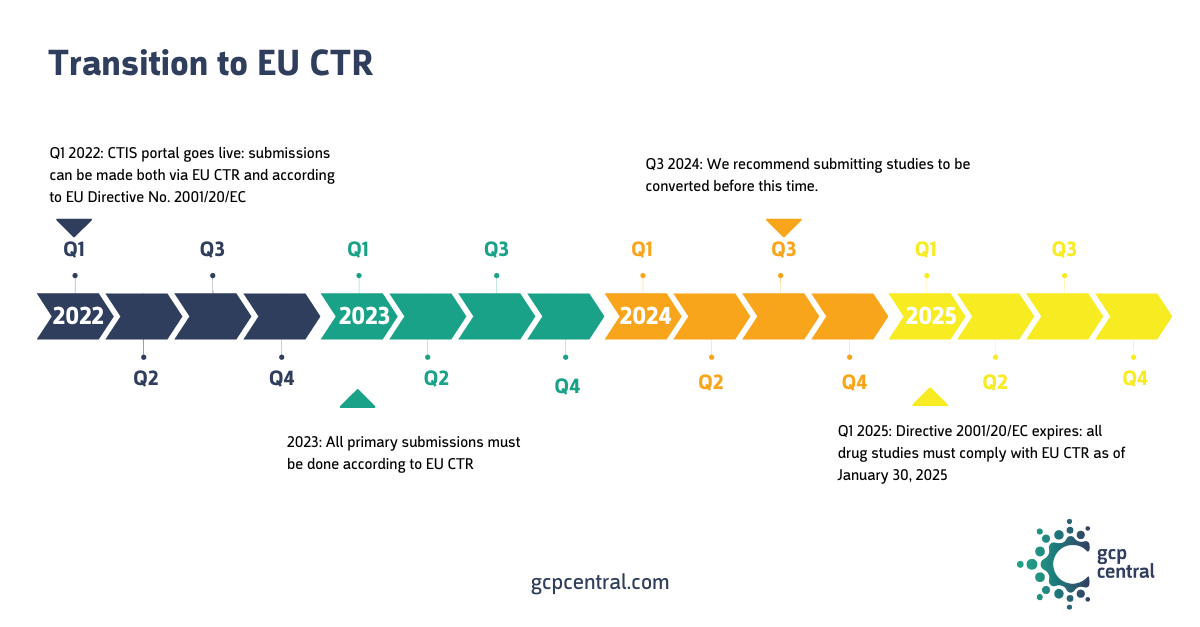
The Clinical Trials Regulation (Regulation (EU) No 536/2014) or, in short, the EU CTR entered into force on January 31, 2022. A three-year transition period was established to allow for a smooth transition of ongoing studies. Thus, on January 30, 2025, all ongoing studies must meet the EU CTR requirements and be included in the Clinical Trial Information System (CTIS). Drug studies authorized under the EU Directive No 2001/20/EC must be terminated or converted by then.
Please answer the following questions for yourself:
- Is your drug study authorized under EU Directive N0 2001/20/EC?
- Is the expected end date after January 30, 2025? Are research sites still active in a European member state or a country in the EEA after January 30, 2025?
- Are any substantial amendments or approvals required for that research?
If the first “yes” is followed by another “yes,” then your research must be converted to the CTR in 2024. This is an administrative process and will take some time.
In what way and in what time frame?
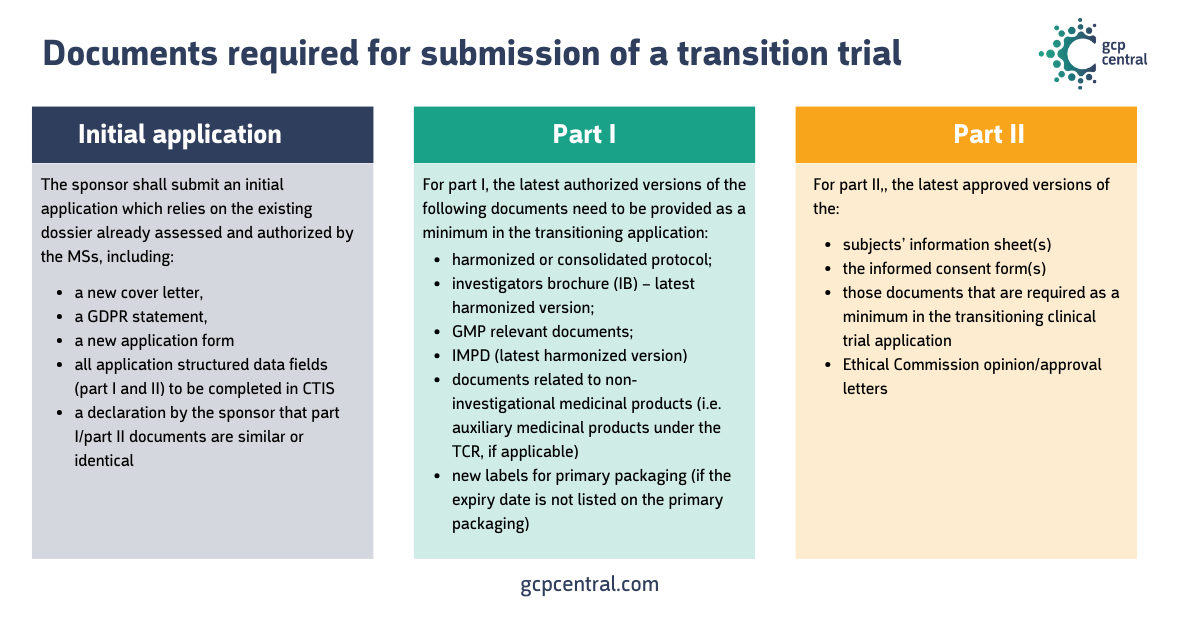
You initiate the conversion as a sponsor by submitting a ‘transition trial’ in the CTIS. Documents that you need to upload fall under the ‘simplified file’, whose deliverables are in the image below:
Please note that:
- documents already submitted, such as the protocol, the investigator’s brochure, and the Investigational Medicinal Product Dossier, should be harmonized or adapted for the different EU member states.
- for multinational trials where documents are harmonized between member states, an amendment must first be submitted under the CRD to approve this document before submitting the transition trial in CTIS.
- this may take some time!
We strongly recommend submitting your studies to be transitioned to the CTR no later than September 2024. The standard timeline for reviewing a transition trial is 60 days. An expedited procedure is available for multinational studies, where the review is estimated to take no more than 22 days. MS review is kept to a minimum and verifies that the submitted file meets the requirements of the CTR. Documents previously reviewed by an ethics committee will not be reviewed for content again.
Two more tips for a smooth transition to the EU CTR:
- Make sure you do not submit documents that are not required. This only generates questions from the reviewing committee and, thus, possible delays.
- If mandatory documents for the EU CTR are requested during the CTIS submission process but were not required under the EU Directive, you may upload an empty document. This way you can continue with the steps of the transition trial file.
In short, no need to panic!. However, action is required if your drug trial still needs to be converted.




